A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The r...

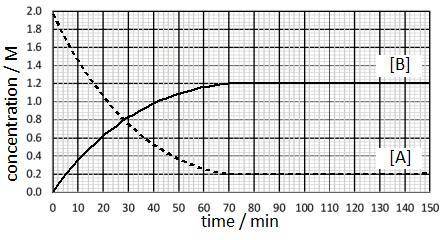
A substance (A) reacts to form another substance (B):
3A(g)
↔
2B(g)
The reaction is run at a particular temperature with the concentrations of A and B monitored over time and plotted in the graph. At what time was equilibrium first reached and what is the approximate value of the equilibrium constant?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

You know the right answer?
Questions

Mathematics, 12.08.2020 05:01


Mathematics, 12.08.2020 05:01



Chemistry, 12.08.2020 05:01





Computers and Technology, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01










