
Chemistry, 09.12.2021 06:20 QueenNerdy889
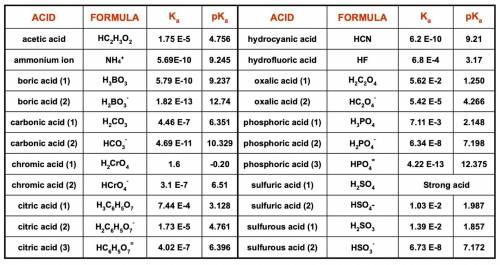
1. List the species present at equilibrium in a solution with the following
composition:
NH4Cl = 0.0200 mol/L NaOH = 0.0430 mol/L
H2SO4 = 0.0150 mol/L NaNO3 = 0.0100 mol/L
2. Write the n equations for n unknowns describing the equilibrium composition of
this system.
3. Make a spreadsheet and use Excel’s Solver function to determine the equilibrium
pH and concentrations of all species in this solution.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1


Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1
You know the right answer?
1. List the species present at equilibrium in a solution with the following
composition:
Questions


Social Studies, 29.03.2020 02:12

Mathematics, 29.03.2020 02:12

Mathematics, 29.03.2020 02:12

Mathematics, 29.03.2020 02:12

English, 29.03.2020 02:12






Mathematics, 29.03.2020 02:13



Mathematics, 29.03.2020 02:13

Arts, 29.03.2020 02:13

Social Studies, 29.03.2020 02:13

Mathematics, 29.03.2020 02:13




