
Chemistry, 09.12.2021 08:40 Savannahpeeler1001
[Review Toples)
In radical chlorination of alkanes, non-equivalent hydrogens react with chlorine atoms at different rates. At 35 °C, primary, secondary, and tertiary C-H bonds
react at relative rates of 1:3.9: 5.2 respectively.
These are conditions of kinetic control where product ratios are determined by relative rates of formation. For example, if A is formed twice as fast as B, the A:B
product ratio will be 2.
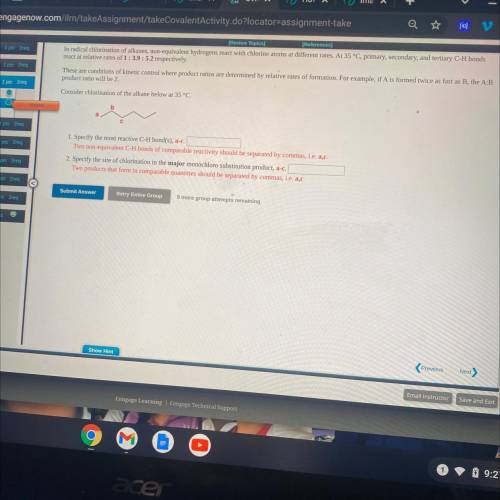
Consider chlorination of the alkane below at 35 °C.
1. Specify the most reactive C-H bond(s), a-c.
Two non-equivalent C-H bonds of comparable reactivity should be separated by commas, i. e. a, c.
2. Specify the site of chlorination in the major monochldro substitution product, a-c.
Two products that form in comparable quantities should be separated by commas, i. e. a, c

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 13:20
Use the periodic table to answer the following questions. what is the predicted order of first ionization energies from highest to lowest for beryllium, calcium, magnesium, and strontium? o be > ca > mg > sr o be > mg > ca > sr o ca > sr> be > mg o sr > ca > mg > be done
Answers: 1

Chemistry, 23.06.2019 15:00
The specific heat of a certain type of cooking oil is 1.75 cal/ (g c) how much heat energy is needed to raise the temperature of 2.67 kg of this oil from 23 c to 191 c
Answers: 1

Chemistry, 23.06.2019 16:00
Which part of the mantle is similar to the crust ? (science)
Answers: 3
You know the right answer?
[Review Toples)
In radical chlorination of alkanes, non-equivalent hydrogens react with chlorine a...
Questions

English, 12.02.2021 09:30



Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30






Biology, 12.02.2021 09:30


Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30

Mathematics, 12.02.2021 09:30


Mathematics, 12.02.2021 09:30




