
Chemistry, 13.12.2021 06:00 ultimatesaiyan
25.0g of iron is heated to 100.0 and then placed in 50.0 g of water in a insulated calorimeter. the initial temperature of the water is 38.00. the specific heat of water is 4.181j/g and the specific heat if iron is 0.45j/g. what is the final temp of the water and the iron?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1



Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
25.0g of iron is heated to 100.0 and then placed in 50.0 g of water in a insulated calorimeter. the...
Questions



Mathematics, 20.04.2020 09:42

Spanish, 20.04.2020 09:42

Mathematics, 20.04.2020 09:42



Mathematics, 20.04.2020 09:42

Mathematics, 20.04.2020 09:42

Mathematics, 20.04.2020 09:42





Mathematics, 20.04.2020 09:43


Mathematics, 20.04.2020 09:43



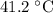 .
.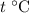 be the final temperature of the water and the iron.
be the final temperature of the water and the iron.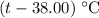 .
.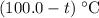 .
. denote the specific heat of each material. Let
denote the specific heat of each material. Let 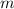 denote the mass of the material. For a temperature change of
denote the mass of the material. For a temperature change of 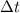 , the energy change involved would be:
, the energy change involved would be: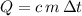 .
.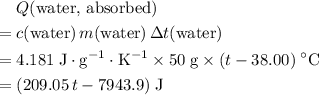 .
.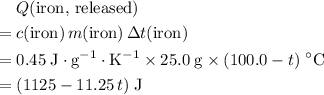 .
.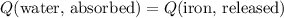 .
.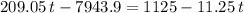 .
.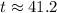 .
.

