-
-
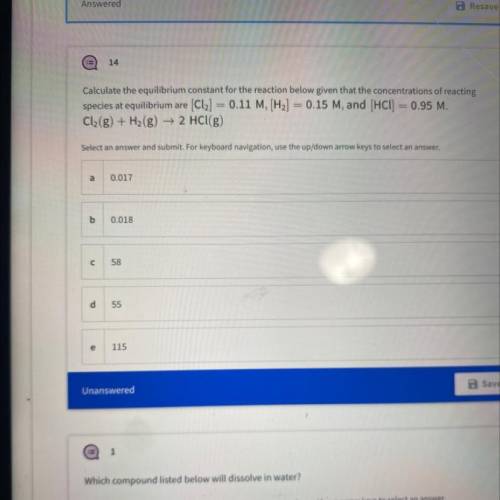
Calculate the equilibrium constant for the reaction below given that the concentrations...

Chemistry, 13.12.2021 06:40 ninigilford
-
-
Calculate the equilibrium constant for the reaction below given that the concentrations of reacting
species at equilibrium are [Cl2] = 0.11 M, [H2] = 0.15 M, and (HCl) = 0.95 M.
Cl2(g) + H2(g) → 2 HCl(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
You know the right answer?
Questions

Mathematics, 05.02.2021 14:00

Physics, 05.02.2021 14:00

History, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00

Physics, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00

Social Studies, 05.02.2021 14:00

English, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00


English, 05.02.2021 14:00


Social Studies, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00


Mathematics, 05.02.2021 14:00

Mathematics, 05.02.2021 14:00


Mathematics, 05.02.2021 14:00




