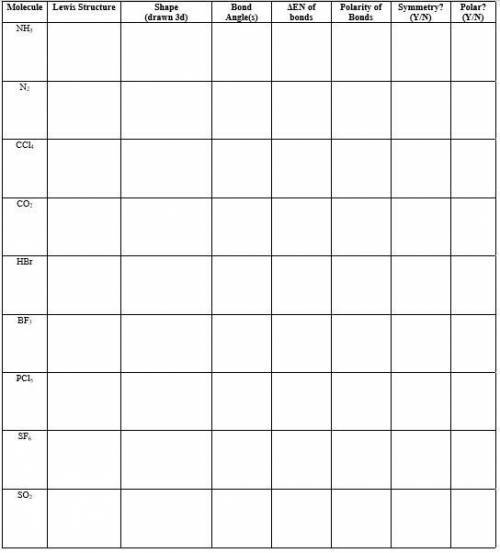
No one in my class understands this please fill it out

Chemistry, 14.12.2021 03:50 oldless504
Please please pleaseee help!!
No one in my class understands this please fill it out

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Please please pleaseee help!!
No one in my class understands this please fill it out
No one in my class understands this please fill it out
Questions


Social Studies, 02.09.2020 03:01

Chemistry, 02.09.2020 03:01


Mathematics, 02.09.2020 03:01




Mathematics, 02.09.2020 03:01


History, 02.09.2020 03:01


History, 02.09.2020 03:01


English, 02.09.2020 03:01

Biology, 02.09.2020 03:01

Geography, 02.09.2020 03:01



Mathematics, 02.09.2020 03:01



