What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g...

Chemistry, 14.12.2021 21:50 baeethtsadia
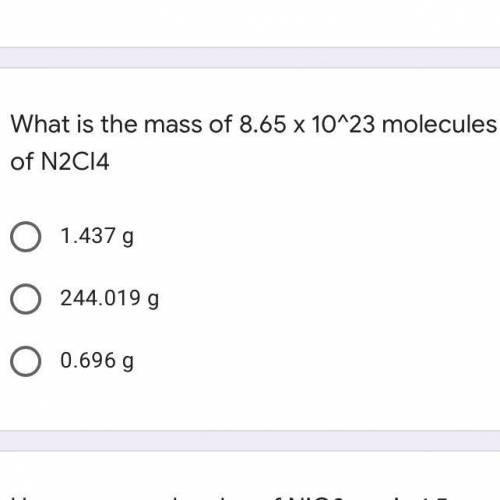
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 10:30
How is it possible for someone to put an ear to a wall and hear someone in the next room? a.sound waves can travel though solids. b.the waves travel from room to room via air. c.there must be some air in the wall so the sound can travel through it. d.sound waves change to electromagnetic waves and then back again.
Answers: 1
You know the right answer?
Questions

History, 21.03.2020 00:18

Biology, 21.03.2020 00:18

Health, 21.03.2020 00:18

Mathematics, 21.03.2020 00:18


Biology, 21.03.2020 00:18

Mathematics, 21.03.2020 00:18





History, 21.03.2020 00:19

Mathematics, 21.03.2020 00:19


Mathematics, 21.03.2020 00:19





Mathematics, 21.03.2020 00:19



