
Chemistry, 14.12.2021 22:00 CaptainKiller528
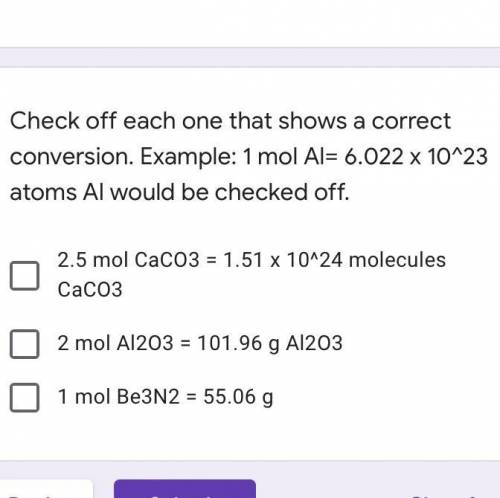
Check off each one that shows a correct conversion. Example: 1 mol Al= 6.022 x 10^23 atoms Al would be checked off.
A. 2.5 mol CaCO3 = 1.51 x 10^24 molecules CaCO3
B. 2 mol Al2O3 = 101.96 g Al2O3
C. 1 mol Be3N2 = 55.06 g

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3
You know the right answer?
Check off each one that shows a correct conversion. Example: 1 mol Al= 6.022 x 10^23 atoms Al would...
Questions


Social Studies, 04.02.2020 03:48

Mathematics, 04.02.2020 03:48



English, 04.02.2020 03:48

Mathematics, 04.02.2020 03:48


Biology, 04.02.2020 03:48



English, 04.02.2020 03:48

Mathematics, 04.02.2020 03:48


Mathematics, 04.02.2020 03:49




Biology, 04.02.2020 03:49

Mathematics, 04.02.2020 03:49



