
Chemistry, 15.12.2021 03:50 juliawatakip5fmg7
The formula for the ionic compound formed between calciumions and chloride ions is CaCl2. What does this formula tell you about the compound? Which one is it :)
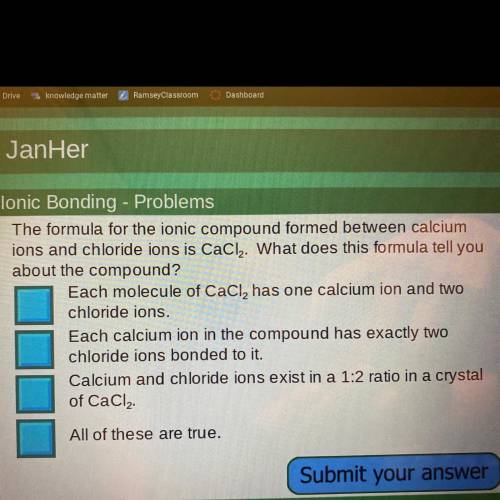

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 11:00
Just on number 2 (all parts), and if you do answer explain in detail
Answers: 3
You know the right answer?
The formula for the ionic compound formed between calciumions and chloride ions is CaCl2. What does...
Questions


History, 12.10.2019 04:30

Mathematics, 12.10.2019 04:30

Computers and Technology, 12.10.2019 04:30



English, 12.10.2019 04:30

Geography, 12.10.2019 04:30

Geography, 12.10.2019 04:30


Chemistry, 12.10.2019 04:30

Mathematics, 12.10.2019 04:30

Mathematics, 12.10.2019 04:30

Biology, 12.10.2019 04:30

Biology, 12.10.2019 04:30



Mathematics, 12.10.2019 04:30




