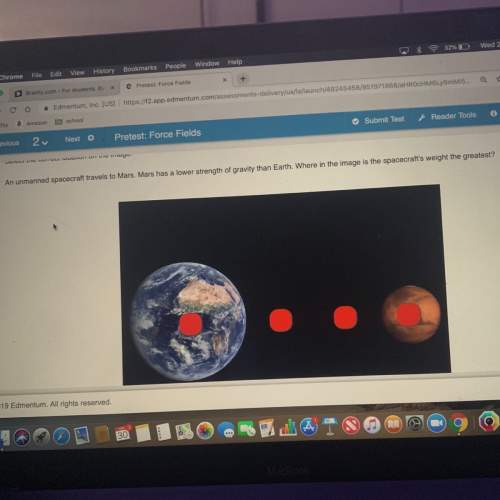
Chemistry, 15.12.2021 19:00 bhopainting
Calculate the number of grams of NH3 produced by the reaction of 6.82 grams of nitrogen with hydrogen. The
chemical equation for this reaction is:
N2 + 3 H2 - 2 NH3

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Calculate the number of grams of NH3 produced by the reaction of 6.82 grams of nitrogen with hydroge...
Questions

Mathematics, 21.04.2021 22:30

Spanish, 21.04.2021 22:30

History, 21.04.2021 22:30



History, 21.04.2021 22:30


Mathematics, 21.04.2021 22:30

Mathematics, 21.04.2021 22:30

Chemistry, 21.04.2021 22:30



Biology, 21.04.2021 22:30



Mathematics, 21.04.2021 22:30

English, 21.04.2021 22:30