
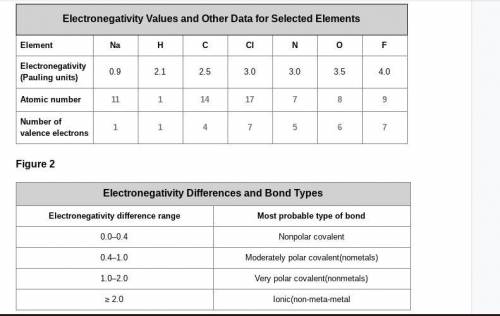
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the data in Figure 1 and Figure 2, what electronegativity value would you expect it to have, and what kind of bond is it likely to form with a chlorine atom? Explain your reasoning.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 23:00
What is formed when amino acids form long chains or polymerize
Answers: 1
You know the right answer?
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the d...
Questions



Advanced Placement (AP), 30.01.2020 01:59


Health, 30.01.2020 02:00

Mathematics, 30.01.2020 02:00


Biology, 30.01.2020 02:00

Social Studies, 30.01.2020 02:00

Health, 30.01.2020 02:00

Social Studies, 30.01.2020 02:00


History, 30.01.2020 02:00


World Languages, 30.01.2020 02:00

History, 30.01.2020 02:00

Geography, 30.01.2020 02:00

History, 30.01.2020 02:00

Mathematics, 30.01.2020 02:00



