
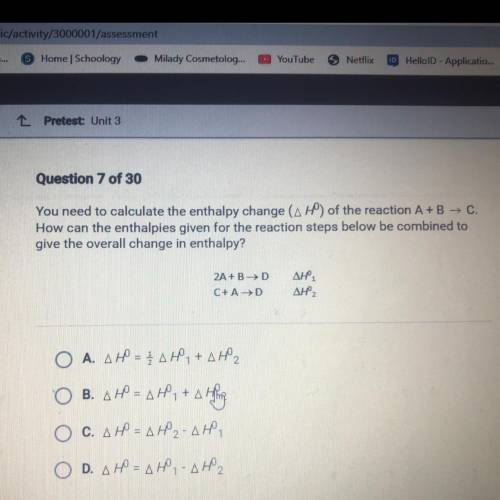
Chemistry, 16.12.2021 23:10 coollid876
You need to calculate the enthalpy change (AHO) of the reaction A+B — C.
How can the enthalpies given for the reaction steps below be combined to
give the overall change in enthalpy?
2A + B HD
С+ AHD
Дне.
ДНР,
ОА. ДР – ДЕ, +д,
ОВ. ДР - ДР. +д Н.
=
Ос. дф = ду-дю,
D. др = два дно,

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
As you move from right to left on the periodic table the atomic radius fill in the blank
Answers: 2



Chemistry, 23.06.2019 05:30
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
You know the right answer?
You need to calculate the enthalpy change (AHO) of the reaction A+B — C.
How can the enthalpies gi...
Questions

Business, 09.07.2019 08:50





History, 09.07.2019 08:50

Social Studies, 09.07.2019 08:50






Mathematics, 09.07.2019 08:50

Mathematics, 09.07.2019 08:50

Mathematics, 09.07.2019 08:50


Social Studies, 09.07.2019 08:50

Biology, 09.07.2019 08:50




