
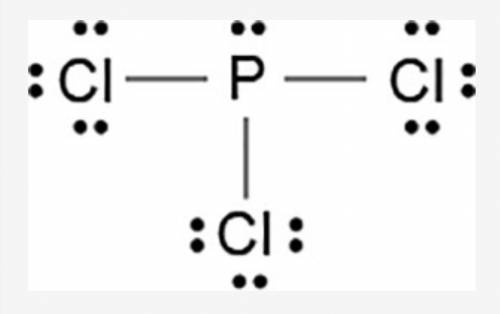
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphorous is the central atom with a horizontal line connecting to each of the three Chlorine atoms around it. Phosphorous has a pair of dots on it. Each of the three chlorine atoms have a pair of three dots on it.
Based on the model, which of the following is true?
The electronegativity difference between phosphorous and chlorine is greater than 1.7.
Each chlorine has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three non-bonded pairs and one bonded pair of electrons.
Phosphorous has three valence electrons in the outermost energy level.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2
You know the right answer?
The Lewis dot model of a molecule is shown.
A visual diagram of a PCl3 molecule is shown. Phosphor...
Questions

Social Studies, 03.10.2019 07:30

Biology, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


English, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


English, 03.10.2019 07:30

Computers and Technology, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30



Mathematics, 03.10.2019 07:30

Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30


Mathematics, 03.10.2019 07:30



