
Chemistry, 20.12.2021 22:10 jesuscruzm2020
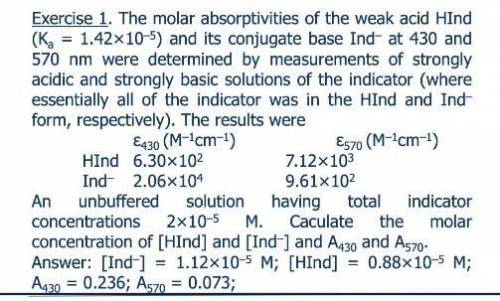
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate base In- were determined by measurements of strongly acidic and strongly basic solutions of the indicator. Under this conditions, essentially all of the indicator was in the HIn and In- form, respectively. The molar ab-sorptivities of HIn and In- at 430 nm and 570 nm were 6.30 ? 102 and 7.12 ? 103, and 2.06 ? 104 and 9.61 ? 102, respectively. Calculate absorbance data for unbuffered solutions that have total indicator concentrations ranging from 2 ? 10-5 to 16 ? 10-5 M.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Supongamos que estás estudiando dos estrellas. ambas estrellas tienen la misma magnitud aparente, pero la estrella a tiene una magnitud absoluta mayor que la estrella b. ¿que puedes decir acerca de la distancia a la tierra de estas dos estrellas?
Answers: 3

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 05:00
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
You know the right answer?
The molar absorptivities at 430 and 570 nm of the weak acid HIn (Ka = 1.42 ? 10-5) and its conjugate...
Questions

History, 03.06.2020 13:14

Biology, 03.06.2020 13:14

Mathematics, 03.06.2020 13:14




Mathematics, 03.06.2020 13:14

Mathematics, 03.06.2020 13:14



Biology, 03.06.2020 13:14

Mathematics, 03.06.2020 13:14



Mathematics, 03.06.2020 13:14




History, 03.06.2020 13:14

English, 03.06.2020 13:14



