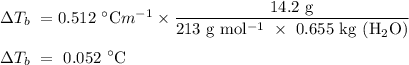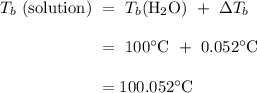Chemistry, 26.12.2021 06:50 haybaby312oxdjli
If 14.2 g of Al(NO_3)_3 is dissolved in 655g of water, what is the boiling point of the solution

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1

Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1

Chemistry, 23.06.2019 15:30
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3+(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1

Chemistry, 23.06.2019 20:10
Sulfonic acids react like carboxylic acids when treated with thionyl chloride. draw the product of the reaction of para-toluene sulfonic acid with thionyl chloride. the name of the product will give you a hint.
Answers: 2
You know the right answer?
If 14.2 g of Al(NO_3)_3 is dissolved in 655g of water, what is the boiling point of the solution...
Questions







English, 26.07.2019 14:30

English, 26.07.2019 14:30

Arts, 26.07.2019 14:30

Arts, 26.07.2019 14:30

English, 26.07.2019 14:30


Computers and Technology, 26.07.2019 14:30



Chemistry, 26.07.2019 14:30

Mathematics, 26.07.2019 14:30



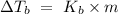 , where m is the molality defined as the number of moles of solute per kilograms of solvent and
, where m is the molality defined as the number of moles of solute per kilograms of solvent and 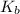 is the molal boiling point elevation constant.
is the molal boiling point elevation constant.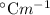 ,
,