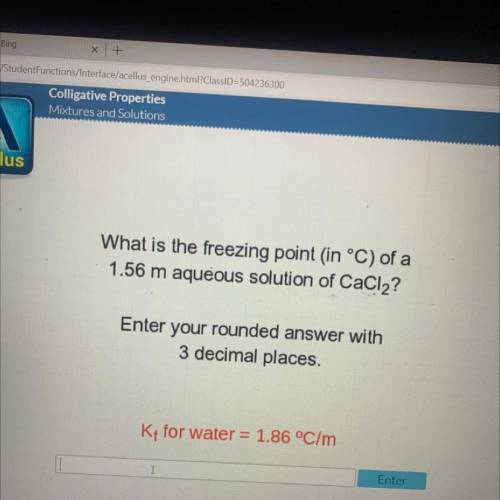
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded...

Chemistry, 02.01.2022 14:00 edimilperdomo
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
Kt for water = 1.86 °C/m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
Questions






Business, 12.10.2020 04:01

Biology, 12.10.2020 04:01

English, 12.10.2020 04:01

Mathematics, 12.10.2020 04:01

English, 12.10.2020 04:01

Biology, 12.10.2020 04:01

Mathematics, 12.10.2020 04:01



Biology, 12.10.2020 04:01

Mathematics, 12.10.2020 04:01

Social Studies, 12.10.2020 04:01



Mathematics, 12.10.2020 04:01



