
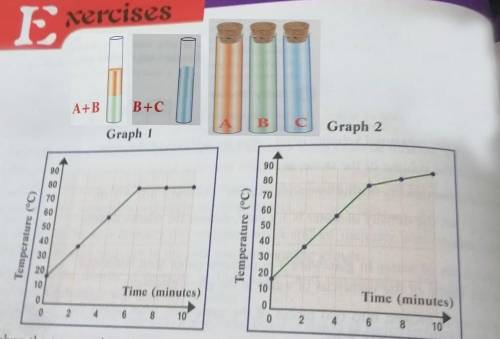
In an attempt to study the variation of the boiling point of mixture (B + C), the teacher immerses a thermometer probe into the mixture inside the test tube. Then, she connects the probe to the computer interface and starts collecting temperature data during the boiling phase. After 10 minutes, a graph is obtained. To which physical state will the above mixture change after boiling? Which of the above graphs do you expect to obtain? why?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
In an attempt to study the variation of the boiling point of mixture (B + C), the teacher immerses a...
Questions

SAT, 08.12.2021 23:10




Social Studies, 08.12.2021 23:10




Mathematics, 08.12.2021 23:10



Arts, 08.12.2021 23:10

Chemistry, 08.12.2021 23:10

History, 08.12.2021 23:10

Biology, 08.12.2021 23:10

Mathematics, 08.12.2021 23:10

English, 08.12.2021 23:10







