
Chemistry, 04.01.2022 21:30 gldenps4780
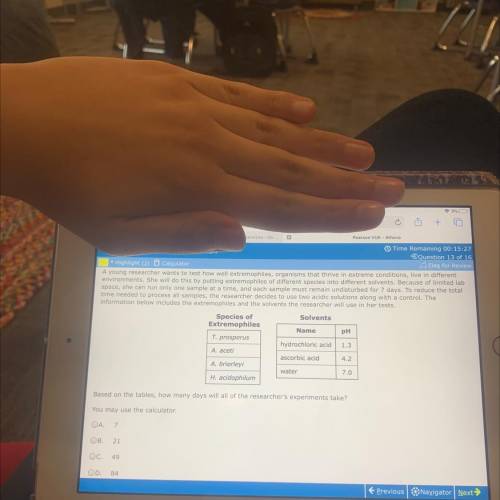
A young researcher wants to test how well extremophiles, organisms that thrive in extreme conditions, live in different
environments. She will do this by putting extremophiles of different species into different solvents. Because of limited lab
space, she can run only one sample at a time, and each sample must remain undisturbed for 7 days. To reduce the total
time needed to process all samples, the researcher decides to use two acidic solutions along with a control. The
information below includes the extremophiles and the solvents the researcher will use in her tests.
Species of
Solvents
Extremophiles
Name pH
T. prosperus
hydrochloric acid 1.3
A. aceti
ascorbic acid 4.2
A. brierleyi
water
7.0
H. acidophilum
Based on the tables, how many days will all of the researcher's experiments take?
You may use the calculator.
A. 7
В.21
C. 49
D. 84

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
A young researcher wants to test how well extremophiles, organisms that thrive in extreme conditions...
Questions





Mathematics, 16.03.2022 14:00


Social Studies, 16.03.2022 14:00





Mathematics, 16.03.2022 14:00




Business, 16.03.2022 14:00




Social Studies, 16.03.2022 14:00



