
Chemistry, 05.01.2022 19:30 natalie857123
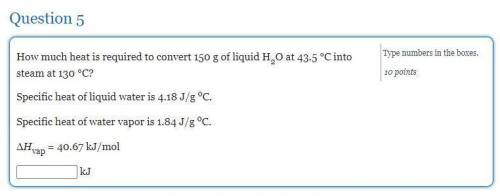
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific heat of liquid water is 4.18 J/g oC.
Specific heat of water vapor is 1.84 J/g oC.
ΔHvap = 40.67 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1
You know the right answer?
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific...
Questions



Mathematics, 14.05.2021 04:30

Mathematics, 14.05.2021 04:30

Biology, 14.05.2021 04:30

Biology, 14.05.2021 04:30

Geography, 14.05.2021 04:30




Biology, 14.05.2021 04:30

English, 14.05.2021 04:30

History, 14.05.2021 04:30





Mathematics, 14.05.2021 04:30

Mathematics, 14.05.2021 04:30

Mathematics, 14.05.2021 04:30



