Chemistry, 12.01.2022 14:00 emmaogle2003
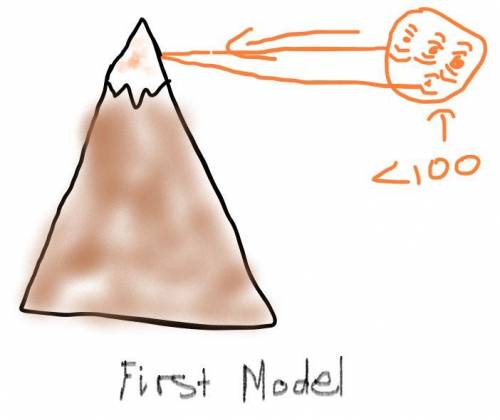
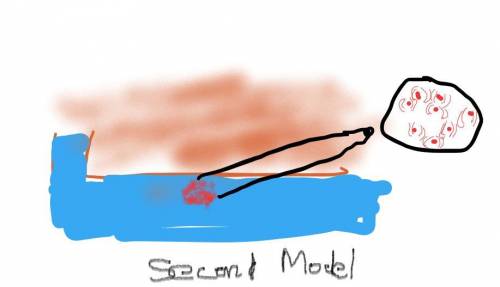
Air pressure decreases as elevation increases. Identify how the boiling point of water on top of a mountain would be different from its boiling point at sea level. Draw two models to show how the particle motion and the state of water on the mountain top and at sea level would change if you kept adding thermal energy to water that was already at 100°C. Label your models to show what happens to the temperature as the energy is added.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 22:00
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1

Chemistry, 22.06.2019 22:30
[ou.03jthe pictures below show the wavelengths and intensities of electromagnetic radiations emitted by three stars, star 1, star 2, and star 3. intensity intensity- intensity- 1000 3500 6000 8500 11000 wavelength (a) star 1 1000 3500 6000 8500 11000 1000 3500 6000 8500 11000 wavelength (a) wavelength (a) star 2 star 3 which of these statements is correct about the color of the three stars? star 2 is white in color o star 2 is yellow in color star 1 and star 3 are yellow in color star 1 and star 3 are white in color
Answers: 1

You know the right answer?
Air pressure decreases as elevation increases. Identify how the boiling point of water on top of a m...
Questions

Mathematics, 20.09.2020 03:01



History, 20.09.2020 03:01


Chemistry, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01

History, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01


Biology, 20.09.2020 03:01

World Languages, 20.09.2020 03:01





Mathematics, 20.09.2020 03:01


Chemistry, 20.09.2020 03:01

Mathematics, 20.09.2020 03:01