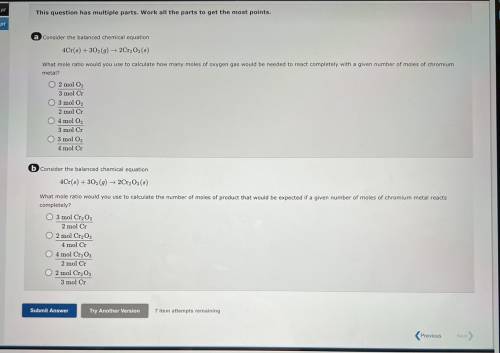
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would yo...

Chemistry, 20.01.2022 19:30 lLavenderl
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would you use to calculate how many moles of oxygen gas would be needed to react completely with a given number of moles of chromium
metal?
O 2 mol O,
3 mol Cr
O 3 mol O2
2 mol Cr
04 mol O
3 mol C
O 3 mol O2
4 mol Cr
6 Consider the balanced chemical equation
4Cr(6) +302 (9) ► 2Cr, Os()
What mole ratio would you use to calculate the number of moles of product that would be expected if a given number of moles of chromium metal reacts
completely?
O 3 mol Cr2O3
2 mol C
O 2 mol CrgO;
4 mol Cr
O 4 mol Cr2O3
2 mol Cr
O 2 mol Cryo,
3 mol Cr

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1

Chemistry, 23.06.2019 14:00
Which statement describes the arrhenius interpretation of acids and bases?
Answers: 1
You know the right answer?
Questions

Mathematics, 28.01.2021 20:10


Mathematics, 28.01.2021 20:10



Mathematics, 28.01.2021 20:10





Mathematics, 28.01.2021 20:10

Mathematics, 28.01.2021 20:10





Mathematics, 28.01.2021 20:10





