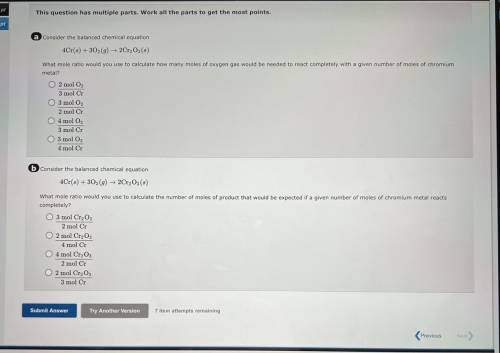
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would yo...

Chemistry, 21.01.2022 01:00 aliciaa101
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would you use to calculate how many moles of oxygen gas would be needed to react completely with a given number of moles of chromium
metal?
O 2 mol O,
3 mol Cr
O 3 mol O2
2 mol Cr
04 mol O
3 mol C
O 3 mol O2
4 mol Cr
6 Consider the balanced chemical equation
4Cr(6) +302 (9) ► 2Cr, Os()
What mole ratio would you use to calculate the number of moles of product that would be expected if a given number of moles of chromium metal reacts
completely?
O 3 mol Cr2O3
2 mol C
O 2 mol CrgO;
4 mol Cr
O 4 mol Cr2O3
2 mol Cr
O 2 mol Cryo,
3 mol Cr

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Questions

Physics, 27.08.2019 16:30



English, 27.08.2019 16:30



History, 27.08.2019 16:30

History, 27.08.2019 16:30

Social Studies, 27.08.2019 16:30


Mathematics, 27.08.2019 16:30



Mathematics, 27.08.2019 16:30




Computers and Technology, 27.08.2019 16:30

Mathematics, 27.08.2019 16:30

Mathematics, 27.08.2019 16:30



