HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au<...

Chemistry, 23.01.2022 14:00 sarahgrindstaff123
HELP PLEASE
Use the following steps to balance the redox reaction below:
Mg + Au+ Mg2+ + Au
a. Write the oxidation and reduction half-reactions. Make sure each half-reaction is balanced for number of atoms
and charge. (3 points)
b. Multiply each half reaction by the correct number in order to balance charges for the two half reactions
c. Add the equations and simplify to get a balanced equation
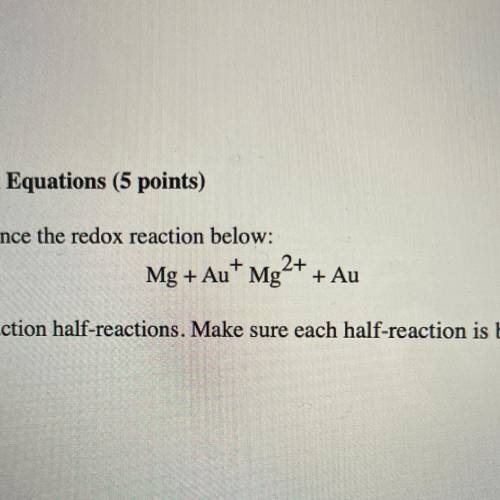

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
Questions


Chemistry, 28.01.2021 19:40

World Languages, 28.01.2021 19:40



Mathematics, 28.01.2021 19:40

Mathematics, 28.01.2021 19:40

Social Studies, 28.01.2021 19:40

History, 28.01.2021 19:40


Mathematics, 28.01.2021 19:40


Mathematics, 28.01.2021 19:40




Spanish, 28.01.2021 19:40

Social Studies, 28.01.2021 19:40


Chemistry, 28.01.2021 19:40



