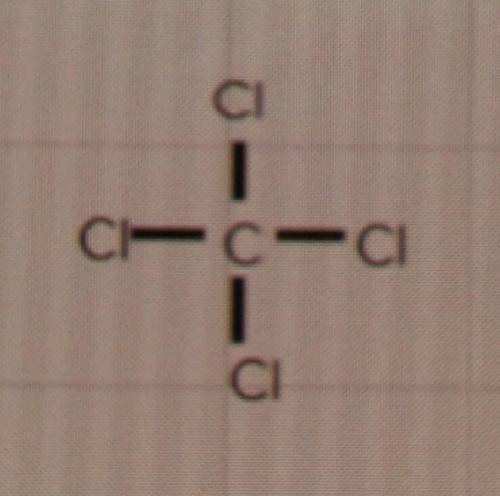Chemistry, 23.01.2022 20:50 Ilcienne6590
Hi! Can someone help me with this???
The question: Will these compounds form single, double or triple bonds?
d) CCℓ4
I'm just a bit confused because the Lewis structures look like it would be a 4 bond, but I don't know if thats even a thing. My only options are single, double or triple.
Thank you!

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Hi! Can someone help me with this???
The question: Will these compounds form single, double or tri...
Questions


Mathematics, 02.07.2019 02:30

Mathematics, 02.07.2019 02:30

Spanish, 02.07.2019 02:30

Biology, 02.07.2019 02:30

Mathematics, 02.07.2019 02:30


Social Studies, 02.07.2019 02:30




Spanish, 02.07.2019 02:30



Social Studies, 02.07.2019 02:30


Mathematics, 02.07.2019 02:30

Geography, 02.07.2019 02:30