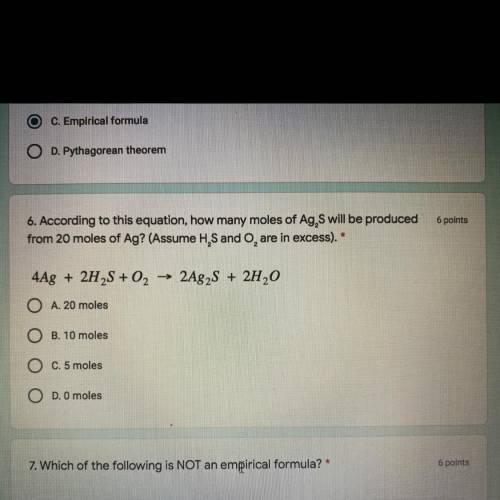
6 points
6. According to this equation, how many moles of Ag, S will be produced
from 20 mol...


Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Questions



Biology, 06.01.2021 04:40

Chemistry, 06.01.2021 04:40

Chemistry, 06.01.2021 04:40






Mathematics, 06.01.2021 04:40



Mathematics, 06.01.2021 04:40


English, 06.01.2021 04:40

Chemistry, 06.01.2021 04:40

Business, 06.01.2021 04:40