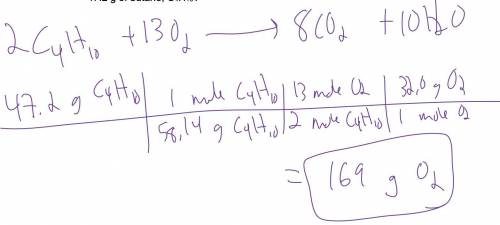Chemistry, 24.01.2022 07:00 christopherandp66l91
How many grams of oxygen are required for the complete combustion of 47.2 g of butane, C4H10? 2C4H10 + 13O2 = 8CO2 + 10H2O

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
How many grams of oxygen are required for the complete combustion of 47.2 g of butane, C4H10?
2C4H...
Questions

History, 17.10.2020 05:01


Mathematics, 17.10.2020 05:01


History, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

English, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Geography, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01

Mathematics, 17.10.2020 05:01



Mathematics, 17.10.2020 05:01


History, 17.10.2020 05:01