Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the o...

Chemistry, 24.01.2022 09:50 hurtadocrv
Cyclobutane decomposes to ethylene according to the equation
C4H8(g) → 2C2H4
Determine the order of the reaction and the rate constant based on the following pressures, which were recorded when the reaction was carried out at 430°C in a constant-volume vessel.
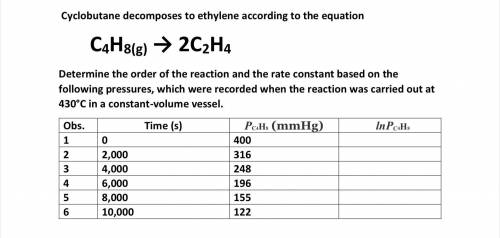

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 03:40
Write the overall equation for the reaction occurring in lithium battery?
Answers: 3
You know the right answer?
Questions

Business, 19.05.2021 14:30



Health, 19.05.2021 14:30

Chemistry, 19.05.2021 14:30


Mathematics, 19.05.2021 14:30


History, 19.05.2021 14:30

Mathematics, 19.05.2021 14:30



Mathematics, 19.05.2021 14:30


Mathematics, 19.05.2021 14:30

Mathematics, 19.05.2021 14:30

Mathematics, 19.05.2021 14:30



Arts, 19.05.2021 14:30



