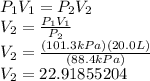Chemistry, 27.01.2022 09:20 glocurlsprinces
A sample of gas has a volume of 20.0 L at 101.3 kPa. If the pressure is changed to 88.4 kPa, what is the new volume of the gas? Please show the work and explain each step. :)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
A sample of gas has a volume of 20.0 L at 101.3 kPa. If the pressure is changed to 88.4 kPa, what is...
Questions


English, 01.04.2021 07:10

Biology, 01.04.2021 07:10



Mathematics, 01.04.2021 07:10


History, 01.04.2021 07:10

Mathematics, 01.04.2021 07:10

Mathematics, 01.04.2021 07:10


Mathematics, 01.04.2021 07:10

Chemistry, 01.04.2021 07:10

Mathematics, 01.04.2021 07:10



Business, 01.04.2021 07:10

Mathematics, 01.04.2021 07:10

Mathematics, 01.04.2021 07:10