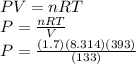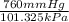Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

You know the right answer?
If I contain 1.7 moles of gas in a container with a volume of 133 liters and at a temperature of 393...
Questions








Biology, 23.06.2019 07:00

Advanced Placement (AP), 23.06.2019 07:00




Mathematics, 23.06.2019 07:00




History, 23.06.2019 07:00

English, 23.06.2019 07:00

Computers and Technology, 23.06.2019 07:00