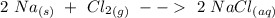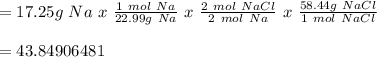Chemistry, 28.01.2022 14:00 mathiscool51
A chemist reacted 17. 25 grams of sodium metal with an excess amount of chlorine gas. The chemical reaction that occurred is shown. Na Cl2 → NaCl If the percentage yield of the reaction is 88%, what is the actual yield? Show your work, including the use of stoichiometric calculations and conversion factors.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

You know the right answer?
A chemist reacted 17. 25 grams of sodium metal with an excess amount of chlorine gas. The chemical r...
Questions

Mathematics, 03.04.2020 08:28

English, 03.04.2020 08:28

Biology, 03.04.2020 08:29


History, 03.04.2020 08:29




History, 03.04.2020 08:29






Biology, 03.04.2020 08:30

Mathematics, 03.04.2020 08:31

Mathematics, 03.04.2020 08:31