
Chemistry, 29.01.2022 01:00 hipstersale4913
20 mins left pls help
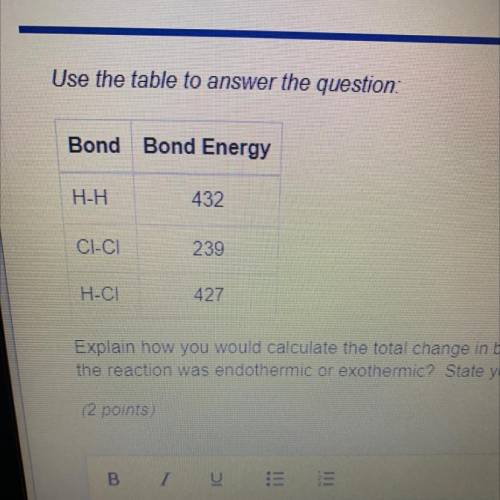
Explain how you would calculate the total change in bond energy for the reaction H2 + Cl2 -> 2HCI How would you know if
the reaction was endothermic or exothermic? State your answer in 3-5 sentences
(2 points)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
20 mins left pls help
Explain how you would calculate the total change in bond energy for the reac...
Questions

Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10

Mathematics, 29.03.2021 20:10

English, 29.03.2021 20:10


Mathematics, 29.03.2021 20:10


Spanish, 29.03.2021 20:10


Mathematics, 29.03.2021 20:20

Mathematics, 29.03.2021 20:20



Chemistry, 29.03.2021 20:20


English, 29.03.2021 20:20


Chemistry, 29.03.2021 20:20


Geography, 29.03.2021 20:20



