
Chemistry, 30.01.2022 02:20 amohammad6
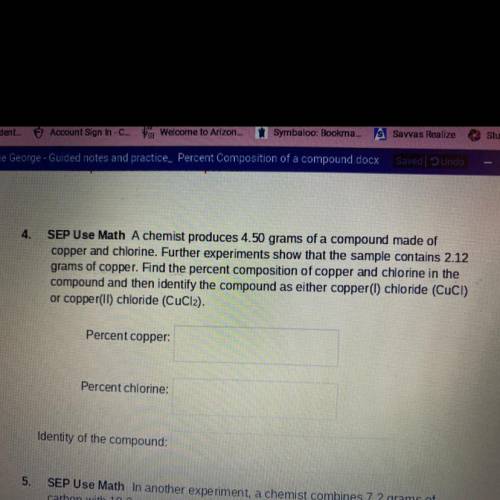
SEP Use Math- A chemist produces 4.50 grams of a compound made of copper and chlorine. Further experiments show that the sample contains 2.12 grams of copper. Find the percent composition of copper and chlorine in the compound and then identify the compound as either copper(I) chloride (CUCI) or copper(II) chloride (CuCl2).

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
SEP Use Math- A chemist produces 4.50 grams of a compound made of copper and chlorine. Further exper...
Questions

Mathematics, 10.03.2021 18:40

History, 10.03.2021 18:40

Mathematics, 10.03.2021 18:40



Physics, 10.03.2021 18:40




History, 10.03.2021 18:40


History, 10.03.2021 18:40

Mathematics, 10.03.2021 18:40

English, 10.03.2021 18:40

Mathematics, 10.03.2021 18:40


English, 10.03.2021 18:40

Biology, 10.03.2021 18:40


Mathematics, 10.03.2021 18:40



