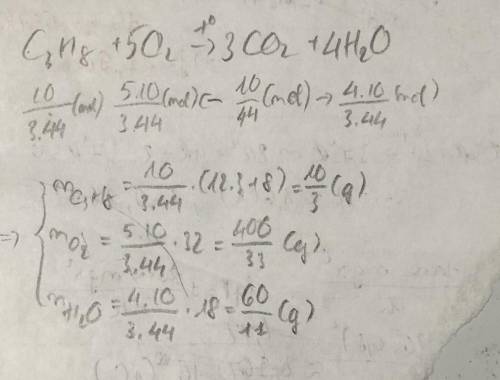Chemistry, 31.01.2022 14:00 Rogeartest4
During combustion reaction of propane (C3H8) the amount of CO2 gas was 10 grams. Calculate the mass of burnt propane, oxygen and water from the reaction? Write and balance the equation. Pls write with explanation)))

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
During combustion reaction of propane (C3H8) the amount of CO2 gas was 10 grams. Calculate the mass...
Questions











Chemistry, 04.05.2021 21:00

English, 04.05.2021 21:00



Mathematics, 04.05.2021 21:00

English, 04.05.2021 21:00


Mathematics, 04.05.2021 21:00

Mathematics, 04.05.2021 21:00

English, 04.05.2021 21:00