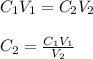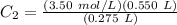Chemistry, 01.02.2022 06:10 probro1167
If 550 mL of a 3.50 M KCl solution are set aside and alowed to evaporate unti the volume of the solution is 275 ml, what will the molarity of the solution be?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3


Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
If 550 mL of a 3.50 M KCl solution are set aside and alowed to evaporate unti the
volume of the so...
Questions



Mathematics, 02.09.2020 06:01

Mathematics, 02.09.2020 06:01

English, 02.09.2020 06:01

English, 02.09.2020 06:01

Biology, 02.09.2020 06:01






Chemistry, 02.09.2020 06:01







English, 02.09.2020 06:01