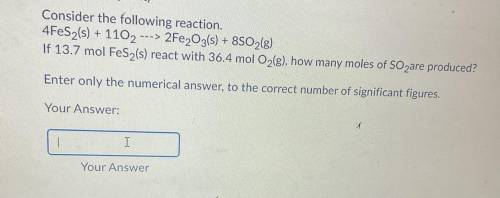
Consider the following reaction.
4FeS2(s) + 1102 ---> 2Fe2O3(s) + 8SO2(g)
If 13.7 mol FeS...

Chemistry, 07.02.2022 21:00 marley5818
Consider the following reaction.
4FeS2(s) + 1102 ---> 2Fe2O3(s) + 8SO2(g)
If 13.7 mol FeS2(s) react with 36.4 mol O2(g), how many moles of SO2 are produced?
Enter only the numerical answer to the correct number of significant figures.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
Questions


Mathematics, 11.02.2020 17:10


Mathematics, 11.02.2020 17:11


Computers and Technology, 11.02.2020 17:11











Law, 11.02.2020 17:11





