
Chemistry, 10.02.2022 20:10 mvasquez3122p4vahv
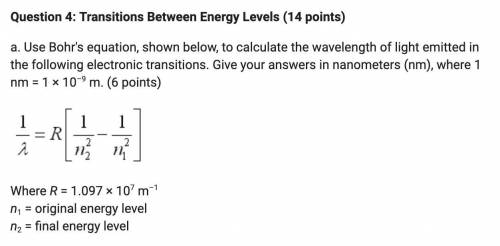
A. Use Bohr's equation, shown below, to calculate the wavelength of light emitted in the following electronic transitions. Give your answers in nanometers (nm), where 1 nm = 1 × 10–9 m.
i. n = 2 n = 1
ii. n = 4 n = 1
iii. n = 6 n = 1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
A. Use Bohr's equation, shown below, to calculate the wavelength of light emitted in the following e...
Questions

Biology, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20






Mathematics, 07.05.2021 18:20





Mathematics, 07.05.2021 18:20


English, 07.05.2021 18:20

History, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20



