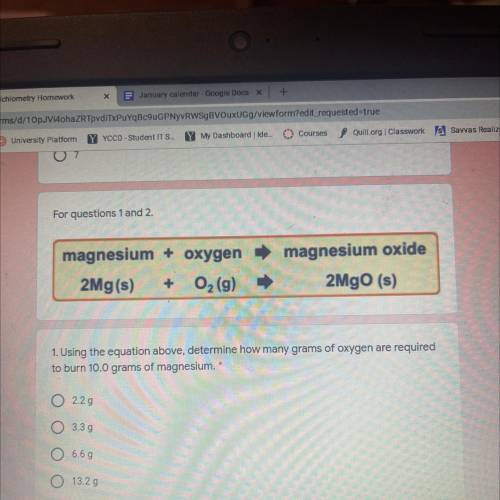
Magnesium + oxygen
2Mg(s) + O2 (9)
magnesium oxide
2MgO (s)
1. Using the equatio...


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which supports the idea that birds and butterflies both have wings but they do not have a common ancestor with wings? a. the wings are analogous structures that evolved differently and do not have a similar internal structure. b. the wings are homologous structures that evolved differently and do not have a similar internal structure. c. wings of birds are vestigial structures, but the wing structures of bats are not vestigial. d. wings of bats are vestigial structures, but the wing structures of birds are not vestigial
Answers: 1

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
You know the right answer?
Questions

Mathematics, 05.09.2020 22:01


Social Studies, 05.09.2020 22:01


History, 05.09.2020 22:01



English, 05.09.2020 22:01


English, 05.09.2020 22:01

English, 05.09.2020 22:01

Mathematics, 05.09.2020 22:01

Mathematics, 05.09.2020 22:01

History, 05.09.2020 22:01

Mathematics, 05.09.2020 22:01


Mathematics, 05.09.2020 22:01



Mathematics, 05.09.2020 22:01