TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactan...

Chemistry, 11.02.2022 22:00 destineenikole17
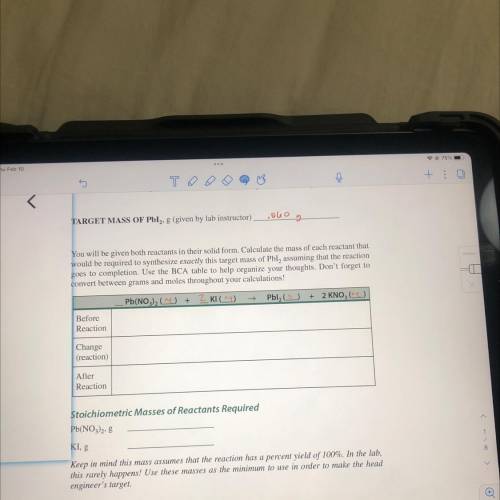
TARGET MASS OF Pbl2, 8 (given by lab instructor)
colo
g
You will be given both reactants in their solid form. Calculate the mass of each reactant that
would be required to synthesize exactly this target mass of Pbl, assuming that the reaction
goes to completion. Use the BCA table to help organize your thoughts. Don't forget to
convert between grams and moles throughout your calculations!
Pb(NO3)2 (
+
2 Klag)
Pbl (5)
+ 2 KNO (2)
Before
Reaction
Change
(reaction)
After
Reaction
I’m

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2
You know the right answer?
Questions

English, 11.02.2020 13:21

Mathematics, 11.02.2020 13:21

Mathematics, 11.02.2020 13:21

Mathematics, 11.02.2020 13:21

Mathematics, 11.02.2020 13:22


Mathematics, 11.02.2020 13:23






Mathematics, 11.02.2020 13:26

English, 11.02.2020 13:28


Mathematics, 11.02.2020 13:39

Mathematics, 11.02.2020 13:39


Mathematics, 11.02.2020 13:45



