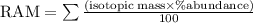Chemistry, 13.02.2022 05:50 starfox5454
Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms are
Indium
−
113
Indium
-
113
(
113
In
113
In
) with a mass of
112.9
u
112.9
u
and
95.72
%
95.72
%
of the atoms are
Indium
−
115
Indium
-
115
(
115
In
115
In
) with a mass of
114.9
u
114.9
u
. Calculate the average atomic mass of Indium with the correct number of significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
Naturally occurring Indium has two isotopes.
4.28
%
4.28
%
of the atoms ar...
%
4.28
%
of the atoms ar...
Questions

Social Studies, 17.06.2021 18:00



Mathematics, 17.06.2021 18:00

Mathematics, 17.06.2021 18:00


English, 17.06.2021 18:00







Mathematics, 17.06.2021 18:00

Mathematics, 17.06.2021 18:00

History, 17.06.2021 18:00

Mathematics, 17.06.2021 18:00

Mathematics, 17.06.2021 18:00