
Chemistry, 13.02.2022 09:30 chippedwood24
Given the following isotopic forms of antimony and their percent abundance, calculate the average atomic mass of antimony with the correct number of significant figures. (submit your work) (Assume that the Mass number, the Atomic number, and the unit conversion factors are all infinite significant figures.)
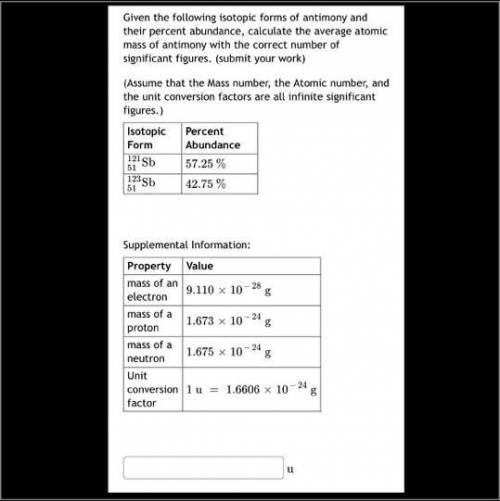

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 1


Chemistry, 23.06.2019 09:20
Four statements about the development of the atomic model are shown below. a: electrons have wavelike properties. b: atoms have small, negatively charged particles. c. the center of an atom is a small, dense nucleus. d: atoms are hard, indivisible spheres. which order of statements represents the historical development of the atomic model? c-d-a-b c-d-b-a d— в-а — с d-b-c-a
Answers: 1
You know the right answer?
Given the following isotopic forms of antimony and their percent abundance, calculate the average at...
Questions


English, 18.01.2022 18:50

Mathematics, 18.01.2022 18:50



Mathematics, 18.01.2022 18:50

Social Studies, 18.01.2022 18:50







Mathematics, 18.01.2022 18:50

SAT, 18.01.2022 18:50

History, 18.01.2022 18:50


Mathematics, 18.01.2022 18:50

World Languages, 18.01.2022 18:50



