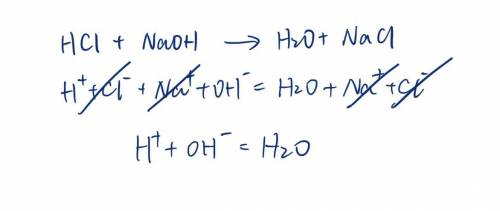Chemistry, 13.02.2022 23:50 ralphmillerrr
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the reaction. Do not include spectator ions in the equation.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
In the reading, yao chen-yuan describes traveling to deliver a message. why was he willing to risk danger to travelto tientsin? he wanted to the boxers with their cause
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
. A strong acid, HCl, is titrated with a strong base, NaOH. Write the net ionic equation for the rea...
Questions




Mathematics, 17.01.2020 06:31

Chemistry, 17.01.2020 06:31

Geography, 17.01.2020 06:31

Mathematics, 17.01.2020 06:31

English, 17.01.2020 06:31

Geography, 17.01.2020 06:31



Business, 17.01.2020 06:31


Geography, 17.01.2020 06:31




Chemistry, 17.01.2020 06:31


Mathematics, 17.01.2020 06:31