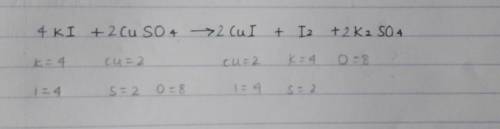(d)
Aqueous potassium iodide reacts with aqueous copper(II) sulfate to produce iodine.
(i) B...


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

You know the right answer?
Questions

Social Studies, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50






Geography, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50




History, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50