
Chemistry, 14.02.2022 09:00 whereswoodruff
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(I) nitrate and barium chloride are combined.
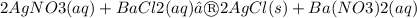
Write the balanced net ionic equation for the reaction.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

You know the right answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions



Arts, 20.04.2020 17:12



English, 20.04.2020 17:13

English, 20.04.2020 17:13

SAT, 20.04.2020 17:13

Mathematics, 20.04.2020 17:13


Mathematics, 20.04.2020 17:13


Social Studies, 20.04.2020 17:13



English, 20.04.2020 17:13

Mathematics, 20.04.2020 17:13


Law, 20.04.2020 17:14




