
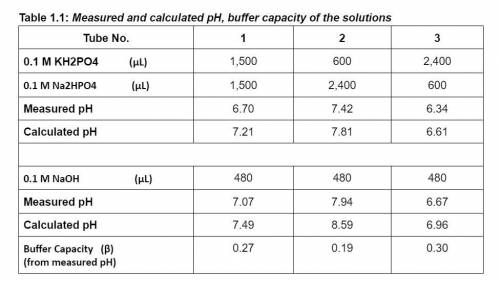
How does the ratio of weak acid and its conjugate base concentrations affect buffer capacity?
Which tube of solutions (molar concentrations of acid and base) is the best to maintain a near-constant pH?
What is the most effective pH range of the buffer based on its pKa value? Why?
How are different between the calculated and experimentally measured pH values of phosphate buffer solutions before and after adding 480 ul of 0.1 M NaOH solution?

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

Chemistry, 23.06.2019 10:30
Most ionic compouds are crystalline solids at room temperature. true falseionic compounds are electrically neutral. true falseionic compounds generally have low melting points. true falsewhen melted, ionic compounds do not conduct electricity. true falsethe electrostatic attraction between an anion and a cation is an ionic bond. true false
Answers: 1

Chemistry, 23.06.2019 11:00
Achemist weighed out 101.g of silver. calculate the number of moles of silver she weighed out.
Answers: 2

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1
You know the right answer?
How does the ratio of weak acid and its conjugate base concentrations affect buffer capacity?
Whic...
Questions

Advanced Placement (AP), 05.03.2021 06:10

Mathematics, 05.03.2021 06:10








Mathematics, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

History, 05.03.2021 06:10

Biology, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10

Health, 05.03.2021 06:10

Mathematics, 05.03.2021 06:10



