
Chemistry, 18.02.2022 07:00 kordejah348
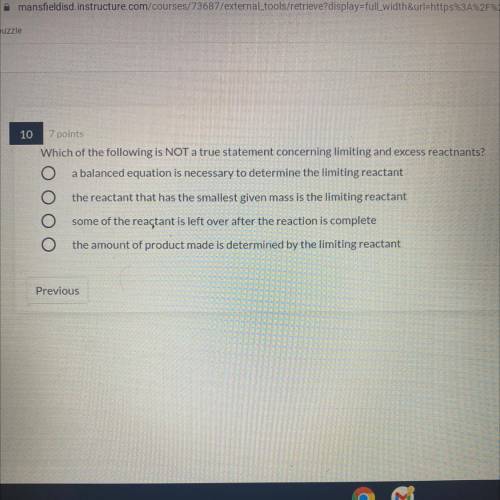
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a balanced equation is necessary to determine the limiting reactant
2.the reactant that has the smallest given mass is the limiting reactant
3. some of the reactant is left over after the reaction is complete
4.the amount of product made is determined by the limiting reactant

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
Which of the following is NOT a true statement concerning limiting and excess reactnants?
1. a bal...
Questions

Mathematics, 26.01.2021 01:00



Health, 26.01.2021 01:00



Geography, 26.01.2021 01:00

Mathematics, 26.01.2021 01:00



Mathematics, 26.01.2021 01:00

Social Studies, 26.01.2021 01:00

History, 26.01.2021 01:00



Mathematics, 26.01.2021 01:00


Mathematics, 26.01.2021 01:00


Mathematics, 26.01.2021 01:00



