
Chemistry, 18.02.2022 08:40 arnold2619
How many moles of aluminum are needed to react completely with 4.8 mol of FeO? 2Al(s) + 3Fe(s) - 3e(s) + Al2O3(s)
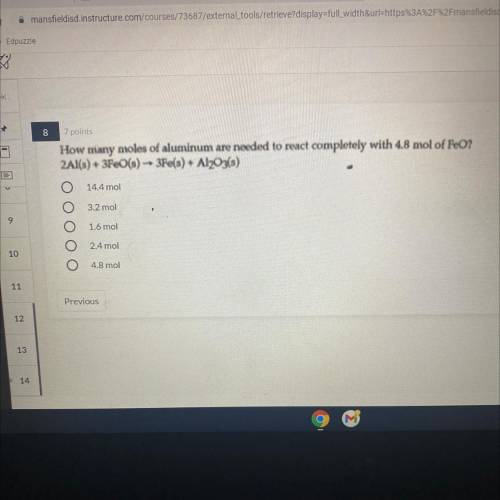

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
How many moles of aluminum are needed to react completely with 4.8 mol of FeO?
2Al(s) + 3Fe(s) - 3...
Questions

Mathematics, 25.07.2019 11:00

Social Studies, 25.07.2019 11:00


Mathematics, 25.07.2019 11:00

Social Studies, 25.07.2019 11:00











Computers and Technology, 25.07.2019 11:00

History, 25.07.2019 11:00

Computers and Technology, 25.07.2019 11:00

History, 25.07.2019 11:00

History, 25.07.2019 11:00



