Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
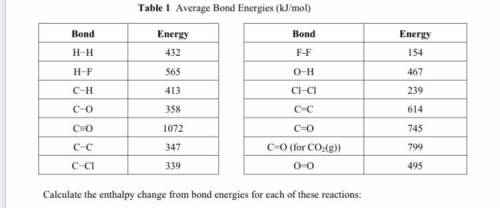
Calculate the enthalpy change from bond energies for each of these reactions:
CO(g) + 2H2(g) → CH3...
Questions



Chemistry, 23.09.2021 06:20

Mathematics, 23.09.2021 06:20

Mathematics, 23.09.2021 06:20






English, 23.09.2021 06:20

History, 23.09.2021 06:20


Mathematics, 23.09.2021 06:20




Social Studies, 23.09.2021 06:20

English, 23.09.2021 06:20