
Chemistry, 21.02.2022 02:00 sofia467735
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for trial 1 and 2.
2. Determine the percent yield of MgO for trial 1 and 2.
3. Determine the average percent yield of MgO for both trials.
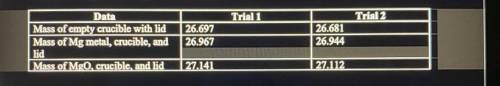

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the total reduction potential of a cell in which potassium (k) is reduced and copper (cu) is oxidized? a. 2.59 v b. 3.27 v c. -3.27 v d.-2.59 v
Answers: 1


Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1
You know the right answer?
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for...
Questions

Mathematics, 06.10.2019 11:40


History, 06.10.2019 11:40


Mathematics, 06.10.2019 11:40

Mathematics, 06.10.2019 11:40



Biology, 06.10.2019 11:40


Mathematics, 06.10.2019 11:40

English, 06.10.2019 11:40

Mathematics, 06.10.2019 11:40

English, 06.10.2019 11:40




Spanish, 06.10.2019 11:40


History, 06.10.2019 11:40



