
Chemistry, 22.02.2022 07:00 vapelordcarl69
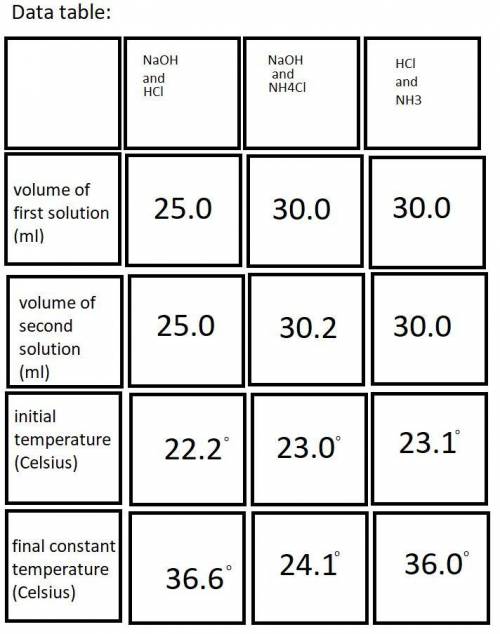
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that heat was lost from the calorimeter, describe specifically how that would impact the data you recorded and then chase that change through the calculations to show how the error would impact the calculated enthalpy change for a particular reaction.
In the past, we have done this lab with roughly 50 mL each of each reactant solution, instead of 25 mL. Yet the Δ T determined experimentally were roughly the same. Explain why that is.
Each student will have slightly different volumes of reactants for each reaction. Yet the results obtained for ΔH1, ΔH2, and ΔH3 should be quite similar and would in fact be quite similar for any range of reactants combined within 25-50 mL, provided that each is accurately measured. Explain why this is the case.
A student misreads the volume of one of the solutions as 25.0 mL when in fact it was 35.0 mL. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated. (Clarification: Both solutions “should” be 25.0 mL or something close, and one of the volumes is accidentally 35.0 mL)
A student pours the reaction solutions into a still wet Styrofoam cup. Step through the quantitative consequences of this error on the heat generated, the Δ T observed, the q calculated, and the Δ H calculated.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
Do you have evidence from the lab that your calorimeter was not a perfect insulator? Assuming that h...
Questions

Biology, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Physics, 18.08.2019 11:30

English, 18.08.2019 11:30

Biology, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Chemistry, 18.08.2019 11:30




Physics, 18.08.2019 11:30

Biology, 18.08.2019 11:30



Mathematics, 18.08.2019 11:30

Business, 18.08.2019 11:30

English, 18.08.2019 11:30



