
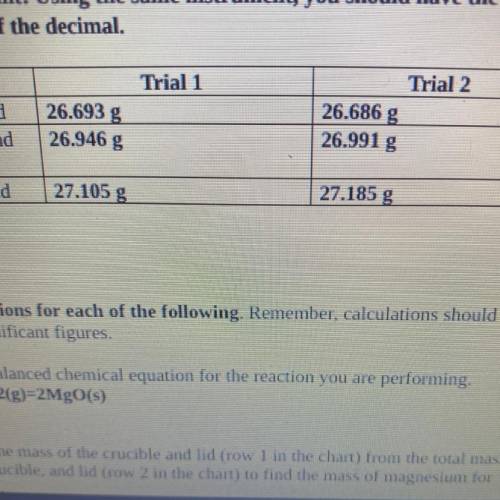
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo for each trial.
. Trial 1:
• Trial 2:
5. Determine the percent yield of Mgo for your experiment for each trial.
Trial 1:
• Trial 2:
6. Determine the average percent yield of Mgo for the two trials.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo f...
Questions


Mathematics, 23.10.2020 21:30

Mathematics, 23.10.2020 21:30

Mathematics, 23.10.2020 21:30

Mathematics, 23.10.2020 21:30


History, 23.10.2020 21:30


Social Studies, 23.10.2020 21:30


Business, 23.10.2020 21:30



History, 23.10.2020 21:30

English, 23.10.2020 21:30



Health, 23.10.2020 21:30




